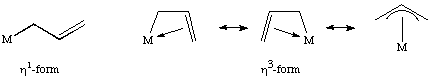Introduction
Allyl ligands are ambidentate ligands that can bind in both a monohapto and trihapto form. The trihapto form can be expressed as a number of difference resonance forms as shown here for an unsubstituted allyl ligand:
Counting electrons in allyl complexes is easy. For the monohapto form, simply consider it to be an alkyl ligand (1 or 2 electron donor depending which formalism you use). In the trihapto form, it is easiest to think of it as an alkyl (1 or two electrons) + a neutral alkene (2 electrons) for a total of 3 or 4 electrons donated.
Structure and Bonding
In the trihapto form, the C-C distances and bond angles are about what we'd expect, 1.35 to 1.40 Angstroms (comparable to that in ferrocene, for example) with a C-C-C angle of 120 degrees. The two C-C bonds are usually the same length, but there are some exceptions, particularly when strongly pi-bonding ligands are trans to the allyl ligand.
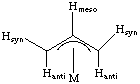
The plane of the allyl ligand is typically tilted away from perpendicular to maximize orbital overlap. Krüger et. al. (Organometallics 1985, 4, 285) report on the neutron diffraction structure of an allyl complex. They found that Hmeso and Hsyn are bent towards the metal (7 and 13 degrees from planar) while Hanti is bent 31 degrees away. In this particular example, the Ni-C1 and Ni-C3 distances are approximately 2.03 Angstroms and Ni-C2 is 1.98 Angstroms:
Spectroscopic Features of Allyl Ligands
The static 1 NMR structure of a typical trihapto allyl has Hanti at 1 - 3 ppm, Hsyn at 2 - 5 ppm and Hmesoaround 4 - 6.5 ppm. There is no syn-anti proton-proton coupling. In the 13C NMR, the terminal carbons appear between 80 - 90 ppm and the central carbon from 110-130 ppm.
Allyl ligands can be fluxional on the NMR time scale, but we won't discuss an example here.
Synthesis of Allyl Complexes
Most syntheses of trihapto allyl complexes involve the synthesis of a monohapto complex which then displaces a ligand to give the trihapto form. Common methods for the synthesis of allyl complexes include:
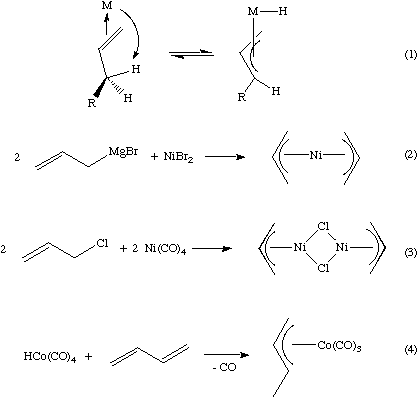
- From an alkene via hydrogen attack on the metal (Eq 1). In the example below, note that if we then put the hydrogen on the other terminal carbon, we have accomplished an overall 1,3-hydrogen shift.
- Nucleophilic or electrophilic substitution at the metal using an allylic substrate (Eq 2)
- Oxidative addition of an allylic substrate to a low valent metal center (Eq 3).
- Protonation or insertion of a 1,3-diene complex (Eq 4).
- There are several other methods as well...
Reactions of Allyl Complexes
All of Chapter 19 of Collman, Hegedus, Norton and Finke is dedicated to reactions of trihapto allyl complexes. Allyl complexes have a rich and useful chemistry, particularly in organic synthesis. Listed here are just a few of the most common reactions of allyl complexes:
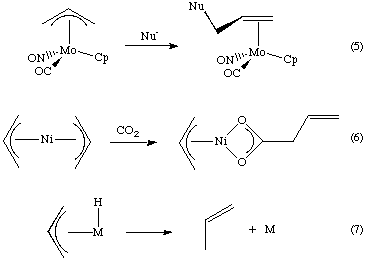
- With nucleophiles (Eq 5). This is stereoselective if the metal is not attacked first. Attack occurs on the face opposite the metal and usually at the terminal carbon.
- With electrophiles.
- Insertion reactions (Eq 6).
- Reductive elimination (Eq 7)

[Index] [Keyword Search] [Books & Software]
[ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.