 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Imido Ligands |
Chemists who work with high valent complexes usually consider the imido unit to be NR2- whereas those that work with low valent complexes tend to consider it a neutral ligand. When specifying how many electrons an imido ligand donates it is therefore necessary to state which electron counting formalism you are using and to consider whether the nitrogen lone pair is participating in the bonding. These are summarized below. Notice the isoelectronic nature of the imido and the oxo ligand:
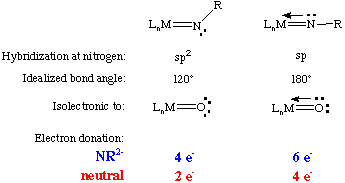
The M-N-R bond angle can, in most cases, be considered as an indicator of the degree of pi-donation from nitrogen. Imido ligands are such good pi-donors that the overwhelming majority of imido complexes are linear or near-linear (bond angles from 150 to 180 degrees) six-electron donors. The only circumstances under which an imido ligand is not linear are:
Imido ligands are of interest not only for their role as ancillary ("spectator") ligands, but for the potential group transfer of the imido unit to an organic moiety

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.