 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| The Monsanto Acetic Acid Process |
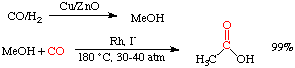
This process has largely replaced an earlier synthetic method called the Wacker process. Over 1,000,000 tons of acetic acid are produced every year using the Monsanto process. The reaction is highly selective, goes in high yield, and is extremely fast. See Adv. Organomet. Chem. 1979, 17, 255 for a good review.
The role of iodide is simply to promote the conversion of methanol to methyl iodide, the species which then undergoes reaction with the Rh metal catalyst:
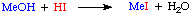
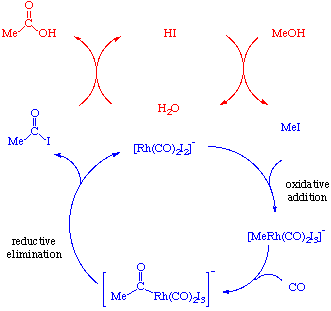
Notice that there are two catalytic cycles going in this reaction. The blue one involves the metal and the red one involves iodide. The acetyl iodide produced in the lower cycle is then hydrolyzed in the upper one to give acetic acid. This hydrolysis produces HI which can then convert more methanol to iodide and continue the cycle:
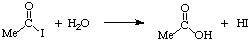
In summary, the system is catalytic in both Rh and I-.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.