 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Phosphine Complexes |
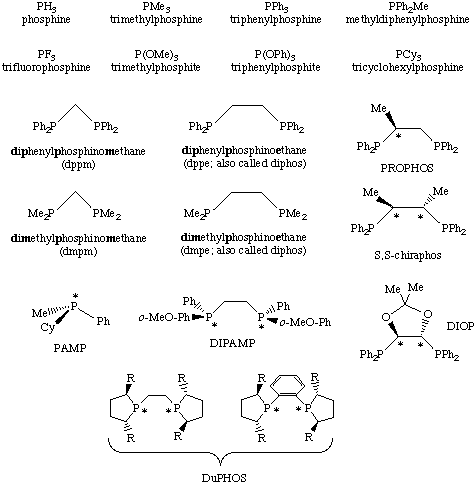
While phosphine ligands are ubiquitous in transition metal chemistry and afford extremely reactive and versatile homogeneous catalysts, a process called phosphine degradation tends to limit their industrial application. Phosphine degradation typically involves an oxidative addition of the phosphine ligand which deactivates the metal center towards further catalysis.

Cone angles for some common phosphine ligands are:
| Phosphine Ligand | Cone Angle |
|---|---|
| PH3 | 87o |
| PF3 | 104o |
| P(OMe)3 | 107o |
| PMe3 | 118o |
| PMe2Ph | 122o |
| PEt3 | 132o |
| PPh3 | 145o |
| PCy3 | 170o |
| P(t-Bu)3 | 182o |
| P(mesityl)3 | 212o |
Take a look at these ball and stick models of various phosphine ligands to get a better idea of their steric attributes. Notice how the phosphites have smaller cone angles than their corresponding phosphines because the oxygen atoms act as "spacers".

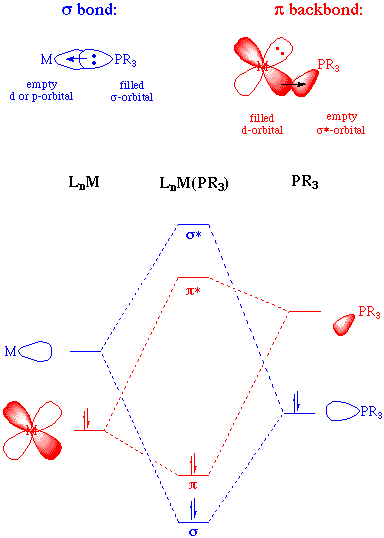
As electron-withdrawing (electronegative) groups are placed on the phosphorous atom, the sigma-donating capacity of the phosphine ligand tends to decrease. At the same time, the energy of the pi-acceptor (sigma-*) on phosphorous is lowered in energy, providing an increase in backbonding ability. Therefore, phosphines can exhibit a range of sigma donor and pi-acceptor capabilities, and the electronic properties of a metal center can be tuned by the substitution of electronically different but isosteric phosphines.
A rough ordering of the pi-accepting or sigma-donating capabilities of phosphines can be accomplished by synthesizing a series of complexes in which the only difference is the nature of the phosphine ligand. If these complexes contain a carbonyl ligand, then the CO stretching frequency can be used as an indicator of electron density at the metal (the lower the value of the CO stretching frequency, the greater the backbonding to the metal and thus the higher the electron density at the metal). Experiments such as this permit us to come up with the following empirical ordering:
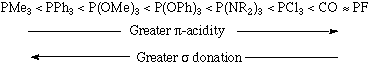

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.