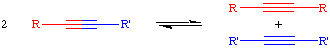General Information
The acetylene metathesis reaction is closely related to the olefin metathesis reaction. The acetylene metathesis reaction can be thought of as one in which all the carbon-carbon triple bonds in a mixture of acetylenes (alkynes) are cut and then rearranged in a statistical fashion:
Acetylene metathesis is not commonly performed and is not used in industry for two primary reasons. First, acetylenes are easily and cheaply made using other methods. Second, competing side reactions (examples below) are unavoidable.
Mechanism
The commonly accepted mechanism for the acetylene metathesis reaction parallels the Chauvin mechanism for olefin metathesis. A [2+2] cycloaddition reaction between a transition metal alkylidyne complex and the acetylene leads to an intermediate metallacyclobutadiene. This metallacycle then breaks up in the opposite fashion to afford a new alkylidyne and a new acetylene. If this process is repeated enough times, eventually an equilibrium mixture of acetylenes will be obtained.
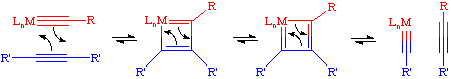
It should be noted here that reaction of acetylenes with alkylidenes leads to the formation of conjugated polymers.
Complications
Schrock has reported several catalytic systems for acetylene metathesis, but competing side reactions are still a major problem. Here are just two examples:
- Formation of deprotio metallacycles: (Schrock et. al., J. Am. Chem. Soc. 1985, 107, 5987). The deprotiometallacycle shown can undergo further insertion reactions with additional equivalents of acetylene.
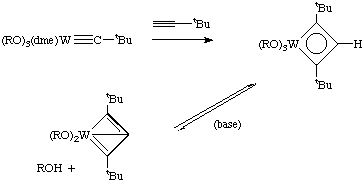
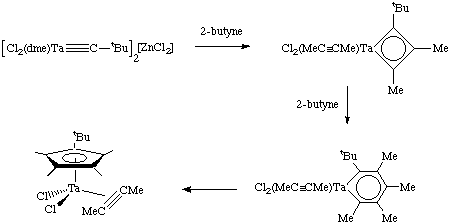
- Formation of cyclopentadienyl complexes (Pasman et. al. Organometallics, 1985, 4, 1847). This turns out to be a novel way of making substituted Cp rings.

[Index] [Keyword Search] [Books & Software]
[ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.