 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Alkylidene Complexes |
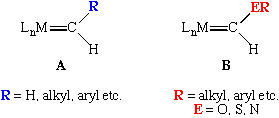
These are sometimes called Schrock carbenes or Schrock alkylidenes in honor of R. R. Schrock who discovered the first example, Ta(CH-t-Bu)(CH2-t-Bu)3, in the 1970's.
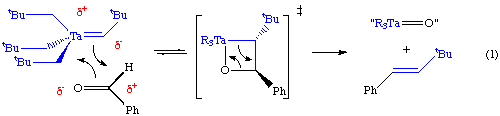
Schrock carbenes are typically found on high oxidation state metal complexes (early to mid transition metals). This polarizes the metal-carbon double bond so that a partial negative charge can be assigned to the alpha carbon atom. Hence, Schrock alkylidenes tend to be nucleophilic at the alpha carbon, an example of which is their Wittig-like reaction with ketones and aldehydes (Eq 1). As one moves to the right in the periodic table, the oxophilicity (propensity for binding to oxygen) of the transition metal decreases, making this reaction less favored.
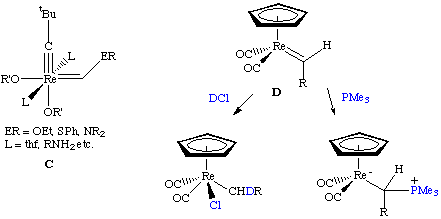
Schrock alkylidene complexes are usually electron deficient or contain strong pi-donating ligands. Contrast this to Fischer carbenes which are typically low valent, low oxidation state complexes containing strong pi-acid (acceptor) ligands such as CO. However, the distinction is sometimes blurred. There are now examples of high oxidation state heteroatom-substituted alkylidenes that do not react like carbenes (C) as well as carbenes that are both electrophilic and nucleophilic (D):
An unusual aspect of alkylidene complexes is a distortion wherein the M-C-C angle is typically much greater than the 120 degrees predicted by a simple sp2 hybridization. Typical M-C-C angles are in the range of 160 to 170 degrees! This distortion has been attributed to an agostic interaction of the alpha proton with the metal as shown below. It can be thought of as the M-H electron pair donating into a vacant d-orbital on the metal center and is most common for electron-deficient complexes (especially for tantalum alkylidenes):
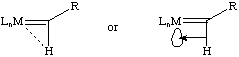
Spectroscopic evidence for this distortion can be found in JCH. A typical sp2 carbon atom has a JCH of 160 Hz. In these distorted alkylidenes, JCHis typically around 120 Hz and can be as low as 90 Hz when the distortion is severe. At this point, the alkylidene looks more like an alkylidyne-hydride complex than an alkylidene. Infrared spectroscopy will often show a C-H stretch of reduced frequency for the alpha proton (2600 cm-1 is typical). In cases where the site for the agostic interaction is blocked, JCH values of 150-160 Hz are typically found and the alkylidene is relatively undistorted.
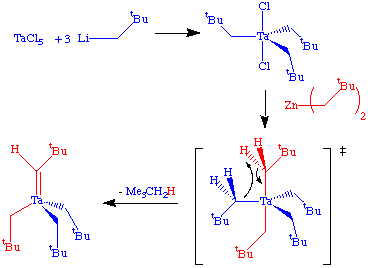
The putative intermediate in this reaction is pentakis(neopentyl)tantalum(V), but this has not been observed spectroscopically. Xue (U of Tennesee) has reported spectroscopic observation of this critical , but reactive intermediate in a silyl analog.
Other methods have also been used such as reduction of alkyl halide complexes and transfer of an alkylidene from a phosphorane or another metal complex (For example, Grubbs has used these methods to synthesize his olefin metathesis catalysts). Reaction of a Wittig reagent (R3P=CR'2) with a metal oxo complex is not a common reaction; apparently the metal-oxo bond strength is too favorable.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.