 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Alpha-Hydride Elimination and Abstraction |
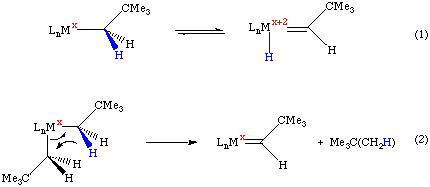
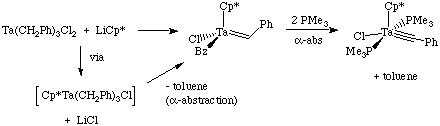
Alpha-abstraction is a common method for synthesizing high oxidation state alkylidene and alkylidyne complexes. While alpha-elimination requires an open coordination site on the metal complex, alpha-abstraction does not. As shown below, alpha-abstraction may be induced by a steric crowding of the metal; the abstraction can occur spontaneously or be induced by addition of a donor ligand such as a phosphine:
The mechanism of both alpha-elimination and alpha-abstraction proceeds through the same sort of four-center transition state as beta-hydride elimination or sigma bond metathesis. Alpha elimination/abstraction can occur when the metal contains both alpha and beta hydrogens, but is most common when beta-hydride elimination is blocked.
The microscopic reverse of a alpha-hydride abstraction would be a called a carbon-hydrogen bond activation in which the C-H bond adds across a metal-ligand bond. In practice, such activations are rare, although highly desirable from the context of functionalization of hydrocarbons.
A third type of elimination reaction encountered with alkyl ligands is the delta elimination reaction.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.