 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Beta-Hydride Elimination |
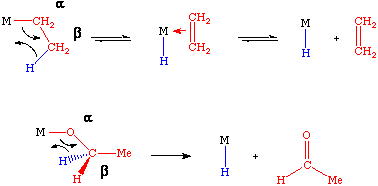
The mechanism shown indicates a four-center transition state in which the hydride is transferred to the metal. In some cases, the unsaturated fragment that is formed will remain bound to the metal and in other cases it will either not bind or be displaced by a stronger donor ligand.
An important prerequisite for beta-hydride elimination is the presence of an open coordination site on the metal complex. If no open site is available, then it will be necessary to displace a ligand before the reaction can occur. In addition, the metal complex will usually have less than 18 electrons, otherwise a 20 electron olefin-hydride would be the immediate product.
The microscopic reverse of a beta-hydride elimination is called an olefin insertion reaction. In many systems there is a rapid, reversible equilibrium between the alkyl complex and the alkene-hydride that results from beta-elimination. Beta-elimination is a very important chain termination step in the polymerization of olefins, in the hydrozirconation reaction and in a variety of catalytic processes.
In order to prevent beta-elimination from taking place, one can use alkyls that:
A few of these are shown below:
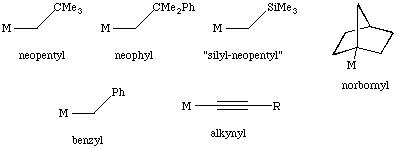
While these approaches can successfully block beta-hydride elimination, the metal complex may still be able to undergo other intramolecular reactions such as alpha-elimination or delta elimination.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.