 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Hydride Complexes |
It should be noted that a bridging hydride does not contain a linear M-H-M angle. This can be rationalized by the bonding description shown here. Conceptually, a bridging hydride is a three-center two-electron bonding situation. We need not need to invoke a metal-metal bond in this description.
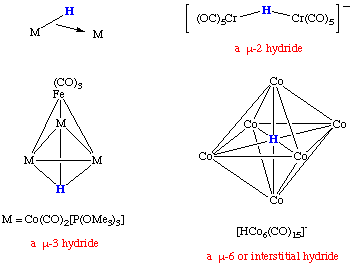
Terminal M-H bonds are observed in the region of 1600 to 2250 cm-1 by infrared spectroscopy while M-H-M bonds are typically found in the region from 800 to 1600 cm-1. These stretches are sometimes weak, particularly with bridging species. In some cases, the stretch may be unobservable or coupled to another vibrational mode in the molecule. Preparation of the corresponding deuteride complex and comparison with the IR spectrum of the hydride is an excellent means of assigning the presence of a hydride ligand as these shifts can be predicted using a harmonic oscillator approach.
Hydrides are usually easy to see in the proton NMR as their proximity to the metal causes large shieldings or deshieldings. There can be problems observing hydrides when the molecule is fluxional or the hydride is attached to one or metal centers with large quadrapole moments. In general, low temperature investigations can get around these obstacles.
The chemical shift range for hydrides is approximately +25 to -60 ppm. The downfield shifts are most common in d0, d10 and early transition metal cases whereas those with other dn counts and late transition metals tend to be upfield of zero. Coupling to other spin active nuclei such as 31P often makes structural assignments unambiguous.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.