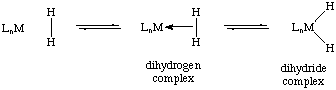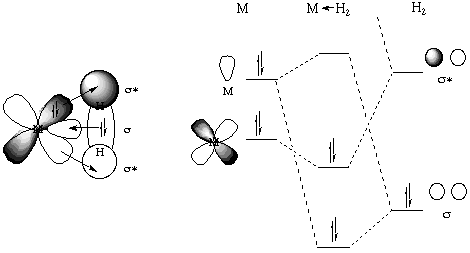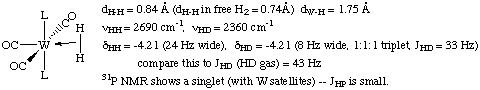General Information
Dihydrogen complexes are transition metal species in which a molecule of molecular hydrogen acts as a two electron sigma donor to the metal center. In this context, the complex is an arrested intermediate in the oxidative addition of dihydrogen to form a metal dihydride. In fact, many dihydrogen complexes exist in equilibria with their dihydride form: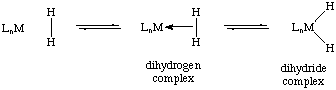
Bonding
The bonding in dihydrogen complexes does not require any unusual explanation. In its simplest sense it is simply a three-center two-electron bond as is commonly seen in boranes. The H2 molecule thus acts a a neutral two electron sigma donor. One could also describe a back-donation of electrons from a filled metal orbital to the sigma-* orbital on the dihydrogen. This is shown schematically in a generic orbital sense (below, left) as well as an MO sense (below, right):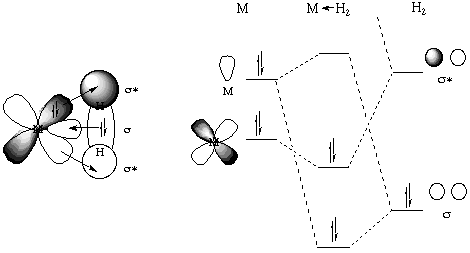
Spectroscopic and Structural Features
The first dihydrogen complex was reported by Greg Kubas of Los Alamos National Laboratory in 1984. A neutron structure was obtained in order to pinpoint the location of the hydrogen atoms: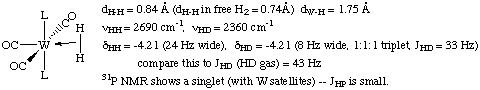
Notice in particular that the H-H bond has been slightly lengthened and that the H-D coupling constant has decreased. However, the H-H distance in this complex is much shorter than in a dihydride complex. Also note that the line width is broadened and the dihydrogen resonance is usually shifted downfield in the 1H NMR of a dihydrogen adduct versus a dihydride.
Another criterion that can be used to help determine whether the complex is a dihydride or a dihydrogen complex is to measure the T1 (spin lattice relaxation time) in the 1H NMR. Although this method has been the subject of some controversy, it is generally agreed that T1 times less than 150 msec are diagnostic of a dihydrogen ligand. The T1 measurement is facile to perform on most modern NMR instruments and requires no special knowledge.
Synthesis
Dihydrogen Complexes are most easily prepared by exposing a precursor compound to hydrogen gas. Typical precursors will have a labile ligand, contain an easily displaced agostic interaction or be less than 18 electrons. Under certain circumstances, dihydrogen complexes can be prepared by the protonation of a hydride ligand. In still other cases, a reversible equilibrium may exist between a dihydride complex and its dihydrogen tautomer.
Further Information
There are dozens of groups involved in this kind of research. For a representative example, take a look at the work of Michael Heinekey.

[Index] [Keyword Search] [Books & Software]
[ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.