 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| The Hydrozirconation Reaction |
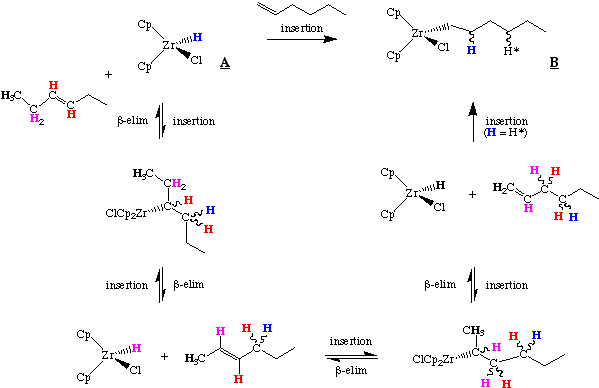
While the scheme above suggests that the final location of the initial Zr hydride (blue) differs depending on whether we start with 1- or 3-hexene, students should convince themselves that this is not necessarily the case; i.e. the hydrogens can scramble.
These insertions and beta-hydride eliminations are quite fast and the olefin is rapidly isomerized to the terminal alkyl form, B. The Zr ends up at the least hindered accessible position in the alkyl chain. This is the thermodynamic product of the reaction and arises primarily from steric interaction with the Cp ring. In the example above, 1-hexene, cis-3-hexene and trans-3-hexene all give the same primary alkyl product. As the migration is driven by steric crowding of the Cp rings, the order of olefin reactivity is about what we would expect:
terminal > cis-internal > trans-internal >exocyclic > disubstituted > trisubstituted
As we also might expect, tetrasubstituted olefins or trisubstituted ones with bulky substituents do not react. The beauty of this reaction is that the resulting Zr-alkyl complex may be derivatized by a variety of reactions: protonation, bromination, insertion, etc., making it a versatile tool in organic synthesis.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.