 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Metal Alkyl Complexes |
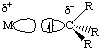
For simple metal alkyls, the M-R bond distance is typically 190 to 220 pm. This is approximately the sum of the covalent radii of carbon and metal, rC = 77 pm and rM ~120 pm. Realize that the first row transition metals are smaller, so any M-X bond distance will usually be smaller by 10-20 pm or so.
Alkyls can bridge two metal centers, something that is well known from aluminum-alkyl chemistry. For example, consider the condensed phase structure of these Al-alkyls (see Oliver et. al. Organometallics 1982, 1, 1307):
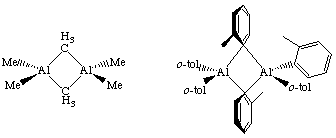
For Al2Me6, we see sharp resonances for bridging and terminal Me groups at -75 degrees C in the 1H NMR, but these coalesce and are one average signal at room temperature. This indicates a very low barrier to interconversion of the two groups. Interestingly, the dimer-monomer equilibrium contains only 0.0047% monomer at 20 ...C!
Bridging alkyls are also known for other metals such as lanthanum and zirconium (see Waymouth, Santarsiero, Grubbs J. Am. Chem. Soc. 1984, 106, 4050-4051):
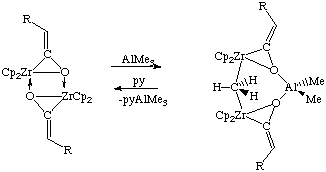
In this structure, the bridging Me group is nearly planar (C is only 8 pm out of the plane defined by the three methyl hydrogens vs. 30 pm for a typical sp3 carbon). The Me bridge is slightly asymmetric (Zr1-C = 259.9(7) and Zr2-C = 245.6(7) pm ) and the Zr-C-Zr angle is 147.8(3) degrees. This non-linearity was ascribed to better orbital overlap on the basis of MO calculations. The JCH = 136 Hz, indicating a fair amount of sp2 character.
Note that while drawing five bonds to carbon makes organic chemists shudder, an MO diagram featuring 3-center-2-electron bonds easily explains the bonding in these complexes.
Another type of unusual alkyl group involves agostic interactions in which part of the alkyl substituent coordinates to the metal in addition to the M-C bond.
Much of this alkylation chemistry can be understood with Pearson's "hard-soft" principles. Here are just a few examples of nucleophilic routes; notice that some of the homoleptic alkyls are rather unstable. This is because they have low d-counts, are susceptible to alpha- and/or beta-hydride elimination, and lack good pi-donating ligands to stabilize their high oxidation states:
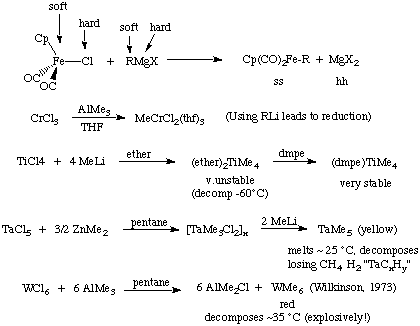
Beware of reactions where the hard-soft interactions are not so clear as these can represent equilibria instead of complete reactions
MCl + AlR3  M-R + AlR2Cl.
M-R + AlR2Cl.
NaFp + RX  Fp-R + NaX [Fp = Cp(CO)2Fe]
Fp-R + NaX [Fp = Cp(CO)2Fe]
One could propose an SN2 (associative mechanism) for this reaction, but a single electron transfer mechanism (SET) could also be postulated.
Whitesides and coworkers examined the above reaction using a stererochemical probe to show that this reaction proceeds with complete inversion of stereochemistry, consistent with an SN2 mechanism. See J. Am. Chem. Soc. 1974 , 96, 2814. However, the nature of the nucleophile is important, and under certain circumstances this can occur through the SET mechanism.
M-X + CH2CH2  M-CH2CH2-X
M-CH2CH2-X
Insertions such as this are involved in the hydrozirconation reaction.
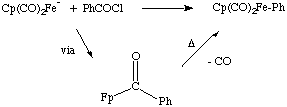
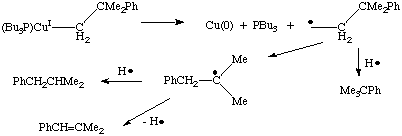
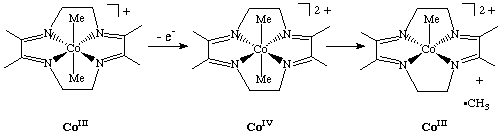
Homolytic cleavage can also be induced by an oxidation reaction that leads to an unstable oxidation state:
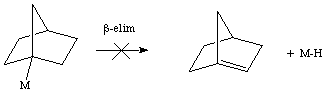
Notice that norbornyl not only has a difficult time approaching the metal center, but that the olefin that would be generated would be highly strained (and violate Bent's rule).
CF bonds are very strong (120-130 kcal/mol vs. 98-104 kcal/mol for alkyl C-H).
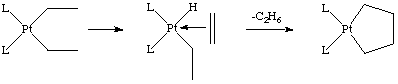
The dialkyl shown on the left decomposes at 110 oC with kdec = 1.0 s-1. In contrast, the metallacycle has k = 5.3 x 10-3 s-1. The beta-hydrogen has a close approach to the metal in the dialkyl case, but not the metallacycle.
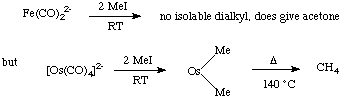
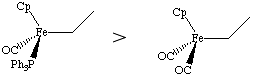
The carbonyl ligand reduces electron density on the metal through pi-backbonding, in contrast to the phosphine ligand, which is a good sigma donor.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.