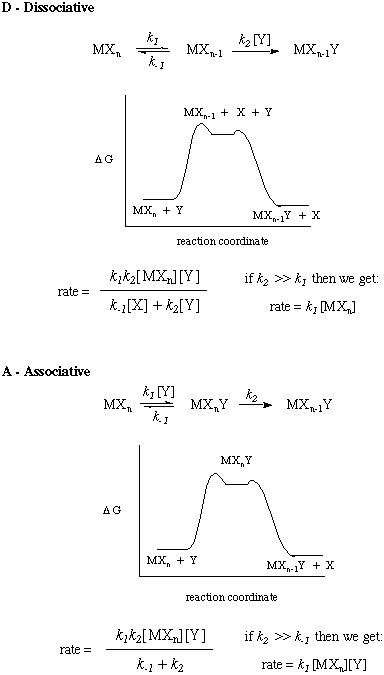Substitution Reaction Mechanisms
When we discuss a substitution reaction in inorganic chemistry we ask ourselves questions about these aspects of the reaction:
- Stoichiometric mechanism -- What are the steps involved? Does a ligand dissociate during the reaction, etc.?
- Intimate mechanism -- What factors specifically affect the rate constant of a particular step? Why is the rate faster for R=Me than for R=Ph etc.?
In particular, we need to ask if a reaction proceeds through an intermediate. This immediately raises the question, "what is an intermediate?" On the potential energy diagram shown below, an intermediate is drawn and connected to the reactants and products by the blue line. But what if the energy barrier to get out of this potential well is so small that we can't detect it? To us, the reaction coordinate might appear not to include an intermediate at all as indicated by the red line: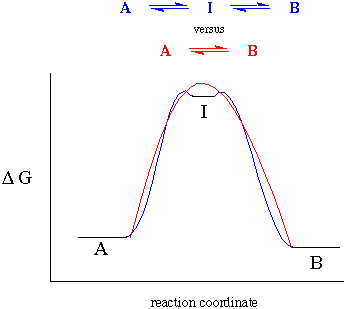
To this end, we will say that for an intermediate to exist we must have some experimental evidence for its existence. This evidence can be a direct observation (by NMR, IR, UV-VIS etc.) or indirect evidence (kinetic analysis).
Classification of Substitution Mechanisms
We can classify reactions into three groups based on their stoichiometric mechanism:
Overall this gives us four limiting cases. A classic organic description of each reaction mechanism is given in parentheses:
- D (comparable to the SN1 limiting case)
- A (comparable to the SN2 limiting case)
- Ia (comparable to typical SN2 reactions)
- Id (comparable to typical SN1 reactions)
Rate Laws for Substitution Mechanisms
The rate laws for different classes of reactions can be derived rather easily. Here are the rate laws and free energy diagrams associated with the mechanisms that we have discussed: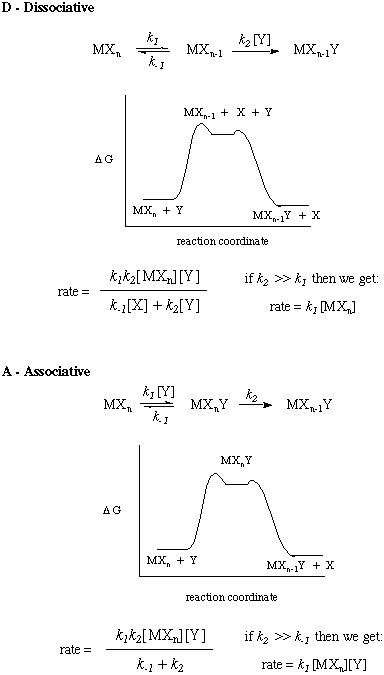
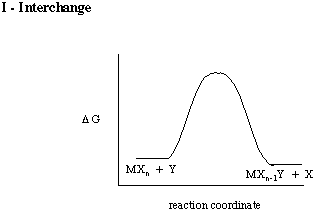
In the case of the Ia mechanism, we could say that the bond from the incoming ligand starts to form before the leaving one starts to break. In the Id mechanism, we could say that an existing metal-ligand bond starts to lengthen or weaken before the incoming ligand arrives. A "pure" interchange would have the leaving ligand-metal bond weaken at the same time that the incoming ligand-metal bond forms.
Additional Resources
- See Andrew Hughes' multi-part lessons on Inorganic reaction mechanisms at the University of Durham.
- Dermont O'Hare has several nice lectures on mechanisms of organometallic reactions. Very well done (slide format).
- Reaction Mechanisms of Inorganic and Organometallic Systems, 2nd Edition
Robert B. Jordan / Hardcover / Published 1998 / 384 pages
Easily understandable advanced undergraduate to graduate-level text dealing with kinetics, ligand substitution, fluxional processes, mechanisms (and skepticism!), electron transfer, photochemistry, experimental methods, solvent exchange, orbital symmetry rules, C-H activation and more. Comes with 900+ references and sample problems.
Approximate price: $60.00
- Inorganic and Organometallic Reaction Mechanisms, 2nd Edition
Jim D. Atwood / Hardcover / Published 1997
A classic text in the area.
Approximate price: $69.95

[Index] [Keyword Search] [Books & Software]
[ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.