 |   |
| Coordination Number and Coordination Chemistry Definitions |
Determining Coordination Number
There are many definitions of the term coordination number, but there is no one simple unambiguous definition that works in all cases. For simple monodentate and chelating ligands, the coordination number can be defined as the number of atoms or ligands directly bonded to the metal atom. For example, [Fe(NH3)6]3+ and [Fe(en)3]3+ are both 6-coordinate complexes. But complications quickly arise when one considers the bonding of alkene or alkenyl fragments such as ethylene and cyclopentadienyl.
For example, in ferrocene all ten cyclopentadienyl carbons are equally bonded to the iron center, but few would call this a 10-coordinate complex. Given the reactivity and bonding of ferrocene, calling it a 2-coordinate complex doesn't seem quite right either. The general consensus is to consider ferrocene a six coordinate species because there are six electron pairs on the ligands that participate in bonding (using the ionic formalism).
While this method seems to alleviate the problem, it adds further complications. Here are some examples:
- Alkoxide, imido and oxo ligands can donate one, two or three pairs of electrons to a metal center, but in all cases each only occupies one coordination site.
- Cp2TiCl2 would not be considered an 8-coordinate complex by most chemists!
- Alkene complexes can be drawn as neutral two electron donors but are sometimes considered dianionic bidentate ligands. Either way, alkenes are usually considered to occupy only one coordination site.
Whew! This isn't easy. The way that I suggest is to count each pair of electrons donated to the metal, ignoring any pi-donation that is localized on a single atom. This allows you to count the pi and delta-type orbitals on cyclopentadiene, but ignore the pi-electrons from an imido ligand.
As far as the number of coordination sites used by a Cp ligand. . . let's just say that it is somewhere between one and three.

Coordinative Unsaturation
We use the term coordinative unsaturation to describe a complex that has one or more open coordination sites where another ligand can be accommodated. Typically, most complexes with a CN of less than 6 fall into this category, although this can vary.
Coordinative unsaturation is an important concept in organotransition metal chemistry. For example, for an olefin to undergo insertion, metathesis or polymerization, there must be room for the olefin to approach or bond to the metal. A common means of creating the requisite vacant site is by loss of a coordinated ligand or a change in the bonding mode of a ligand (such as conversion of a trihapto allyl ligand to the monohapto form). Such changes are often accompanied by a reduction in the electron count as well. Hence, while the terms "coordinative unsaturation" and "electronic saturation" are different, they often work hand-in-hand as part of a catalytic cycle.

Some Definitions
Here's a short glossary of some terms used in coordination and organometallic chemistry:
Ambidentate
Ambidentate ligands can bond to a metal center through more than one kind of atom. The classic example is the NO2- ligand which can bind in a monodentate fashion through the O or the N atom as shown here.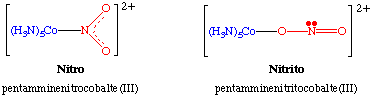
Other examples of ambidentate ligands include SCN-, which can bind through the S (thiocyanato) or through the N (thiocyanate), and sulfoxides, R22S=O, which can bind through either O or S. All three of these ligands are examples of linkage isomerism.
Ambidentate can also refer to a polydentate ligand that is not fully coordinated to a transition metal. For example, ethylenediamine is normally bidentate but may only coordinate through one of the amine groups in certain circumstances.
Bidentate
Bidentate ligands have two points of attachment to the metal center and occupy two coordination sites. Examples of such ligands are 2,2'-bipyridine (bipy), ethylenediamine (en), diphenylphosphinoethane (dppe), acetylacetonate (acac), and oxalate (ox):
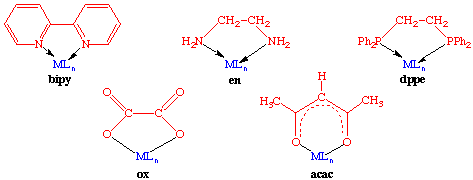
Bite angle
|
A bite angle is the ligand-metal-ligand angle formed when a bidentate or polydentate ligand coordinates to a metal center. This number can be used to characterize the distortion from an idealized geometry or to gauge the degree of ring strain. In the example on the right, we can see that the tetrahedral metal center is distorted from the ideal 109.5o angle. | 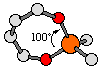
|
Chelating
A chelating ligand (Greek for "claw"), involves a polydentate ligand that forms a ring that includes the metal. Also called a chelate. The examples shown above for bidentate ligands as well as the bite angle example above are additional examples of chelating ligands.
|
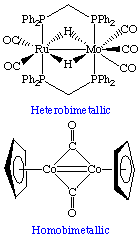
Heterobimetallic
This term describes a complex in which there are two different metal centers, for example, ruthenium (Ru) and molybdenum (Mo).
Homobimetallic
These complexes have two metal centers that are the same element such as cobalt (Co). The two metals centers do not need to have identical ligands or coordination number, but are usually found as symmetric dimers.
|
 |
Homoleptic complexes contain only one kind of ligand.
Ligand
A ligand is a molecule or ion that is bonded directly (i.e. covalently) to a metal center. Organometallic chemists pronounce this (correctly, as we like to say), "Lig-and", while biological chemists pronounce it "lie-gand".
Monodentate
Monodentate ligands have only one point of attachment to the metal center and occupy only one coordination site. Examples of monodentate ligands include NH3, H2O, PMe3, CO and other neutral two-electron donors. A bidentate ligand may act in a monodentate fashion if one end is not attached to the metal center, in which case it is called ambidentate.
Polydentate
Polydentate ligands (also called multidentate ligands) have more than one point of attachment to the metal center and occupy more than one coordination site. By definition, bidentate ligands as well as ambidentate ligands are subsets of polydentate ligands. A 6-coordinate tetraanionic ligand is ethylenediamineteraacetato (edta) shown below in its uncomplexed and complexed states (with hydrogens omitted for clarity). The salen ligand is versatile dianionic, tetradendate ligand that has been exploited by Eric Jacobsen and many others. The tridentate tris(pyrazolyl)borate ligand is an example of a monoanionic tridentate ligand which is often used as a Cp analog.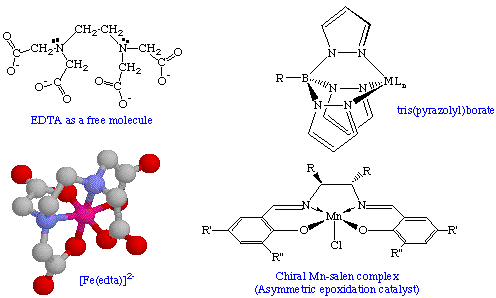
Primary Coordination Sphere
The primary coordination sphere of a metal involves the set of ligands closest to the metal that are directly attached. Mobile cations or counterions are said to be in the outer or secondary coordination sphere.

[Index] [Keyword Search] [Books & Software]
[ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.











