 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Alkene Complexes |
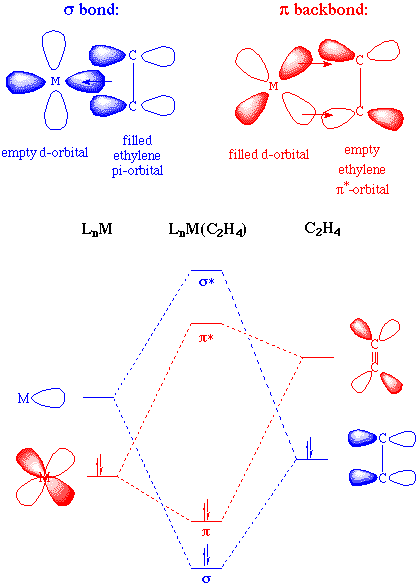
The greater the electron density back-donated into the pi* orbital on the alkene, the greater the reduction in the C=C bond order. An alternative way of stating this would be to say that the hybridization of the alkene carbon changes from sp2 to sp3 as back-donation increases. Either formalism describes two limiting structures: a planar olefin adduct and a metallocyclopropane. X-ray crystallographic studies confirm that the as the C-C bond length increases, the CH2 plane is distorted from the ideal planar geometry of an alkene:
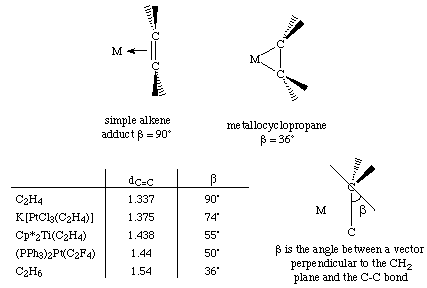
Electronic factors play a large role in the binding of alkenes to transition metals. For example, tetrafluoroethylene will bind more tightly than ethylene to a low valent metal complex because the presence of electron-withdrawing groups on the olefin results in poorer sigma donation and lowers the energy of the pi* orbital (providing better overlap for backbonding). Likewise, ethylene (like carbon monoxide) is a poor ligand for d0 metal complexes because there are no d-electrons to engage in back-bonding.
The stability of alkene complexes also depends on steric factors as well. An empirical ordering of relative stability would be:
tetrasubstituted < trisubstituted < trans-disubstituted < cis-disubstituted < monosubstituted < ethylene.
The reaction chemistry of these complexes is quite broad and could form another textbook in itself. For some other examples of alkene reactivity look at the sections on olefin metathesis, olefin polymerization and insertion reactions.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.