 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Alkyne Complexes |
The primary difference in bonding between alkenes and alkynes is that an alkyne can act as either a 2 or 4 electron donor. Recall that alkynes have two sets of mutually orthogonal pi bonds. We can bind one of these to the transition metal in a sigma-type fashion (A) as we did for alkenes, including a pi-backbond (B). The orthogonal set can also bind in a pi-type fashion using an orthogonal metal d-orbital (C):
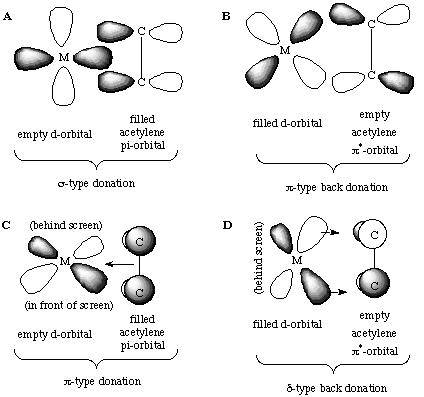
The back-donation to the antibonding orbital (D) is a delta-bond (there are two nodes in it), and the degree of overlap is quite small as the two orbitals meet side-to-side rather than engaging in direct overlap. Therefore, the contribution of D to the bonding of alkynes is minimal at best.
The net effect of this additional pi-donation is that alkynes are usually non-linear when coordinated to a transition metal complex. We can draw several resonance structures that depict the bonding of an alkyne. I is the metallacyclopropene resonance form. Support for this versus a simple two electron donor, II, can be inferred from the C-C bond distance as well the R-C-C-R angles (see below). III generally does not contribute to the bonding of alkyne complexes.
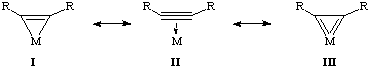
Other than geometry, the other major difference between I and II is that I implies the metal oxidation state is greater by two. In other words, one can sometimes think of alkynes as dianionic ligands instead of as neutral ligands! Remember that electron counting is strictly a formalism.
For a terrific review of 4-electron donor acetylene ligands, see Templeton, J. L. Adv. Organomet. Chem. 1989, 29, 1.
As expected from the reduced C-C bond order, the C-C bond distances for coordinated alkynes are typically larger (125 to 135 pm) than in the uncoordinated ligand (110 to 115 pm).
For 4-electron donors, the R-C-C bond angles are usually in the range of 130 to 146 degrees, with M-C bond distances of 199 to 209 pm.
Finally, it is worth noting that alkynes can also bridge two metal centers. In these cases it is sometimes appropriate to describe the complex as a 1,2-dimetallatetrahedrane. In this case, the alkyne is a 2-electron donor to each metal center:
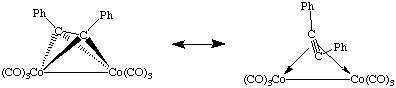
As one might expect, such species can act as a stoichiometric source of benzyne and display a correspondingly rich reaction chemistry. For a terrific review of this area see Jones, W. M.; Klosin, J. Adv. Organomet. Chem. 1998, 42, 147.
Unlike coordinated polyenes and alkenes, nucleophilic attack on a coordinated alkyne is fairly rare. However, rearrangement of terminal acetylene complexes to the vinylidene tautomer is not uncommon:
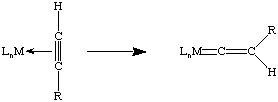
A number of transition metal alkyne complexes are intermediates in the cyclotrimerization of alkynes to substituted benzenes. In some cases, nitriles can be co-cyclized with alkynes to give heteroatom-substituted rings.
The proton of a terminal alkyne usually shifts considerably downfield in the 1H NMR upon coordination to a transition metal, shifting from approximately 1 - 3 ppm to 6 - 14 ppm (10-14 ppm is most typical for 4-electron donors).
The acetylenic carbons also shift downfield in the 13C NMR spectrum, appearing in the range of 100-240 ppm (205 - 225 most typical for 4-electron donors) instead of 60-90 ppm for the free ligand.
Many alkyne complexes exhibit barriers to rotation, as would be expected particularly with a 4-electron donor. Alkyne rotational barriers in such species typically range from 9.1 to 19.9 kcal/mol with 14 - 16 being most common.
IR and Raman
The C-C triple bond of free alkynes are normally observed around 2200 cm-1 in the infrared or Raman spectrum. Coordination to a transition metal reduces the C-C bond order, leading to a lower stretching frequency in the range of 1700 to 2000 cm-1.
4-electron donors reflect their reduced nature in the IR: coordinated internal alkynes are typically 1730 - 1820 cm-1 and coordinated terminal alkynes are typically 1675 - 1715 cm-1.
Remember that symmetric C-C stretches are not IR active, so that an asymmetric alkyne or the use of Raman spectroscopy may be required to observe this spectroscopic feature.

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.