 |   |
| Ring Opening Metathesis Polymerization |
General Information
Ring Opening Metathesis Polymerization (ROMP), a term coined by CalTech chemist Robert Grubbs, is a variant of the olefin metathesis reaction. The reaction uses strained cyclic olefins to produce stereoregular and monodisperse polymers and co-polymers.
Mechanism
The mechanism of the ROMP reaction involves an alkylidene catalyst and is identical to the mechanism of olefin metathesis with two important modifications. First, as the reaction involves a cyclic olefin, the "new" olefin that is generated remains attached to the catalyst as part of a growing polymer chain as is shown below with a generic strained cyclic olefin: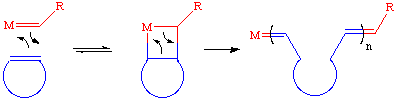
The second difference is that the driving force for the ROMP reaction is the relief of ring strain. Therefore, the second step shown above is essentially irreversible. Olefins such as cyclohexenes or benzene have little or no ring strain and can not be polymerized because there is no thermodynamic preference for polymer versus monomer. Strained cyclic olefins such as those shown below have sufficient ring strain to make this process possible. Monomers based on norbornene derivatives are especially popular as they can be readily synthesized from Diels-Alder reactions with cyclopentadiene. Only the unsubstituted bonds are ring-opened (it is very difficult to metathesize or ROMP tri- and tetrasubstituted olefins).
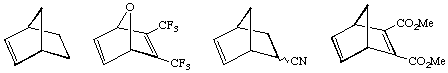
The polymers produced in the ROMP reaction typically have a very narrow range of molecular weights, something that is very difficult to achieve by standard polymerization methods such as free radical polymerization. The polydispersities (the weight average MW divided by the number average MW) are typically in the range of 1.03 to 1.10. These molecular weight distributions are so narrow the polymers are said to be monodisperse.
An important feature of this mechanism is that ROMP systems are typically living polymerization catalysts. For example, one can polymerize 100 equivalents of norbornene and then add a second monomer after the first one is consumed. ROMP is a superior method for making diblock and triblock co-polymers and permits one to tailor the properties of the resulting material.
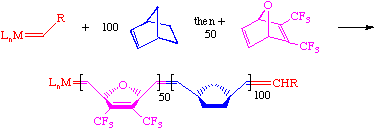
Such techniques are only possible if the ratio of chain initiation and chain propagation are perfectly balanced. Therefore, for functionalized monomers in particular, it is not uncommon to try several different catalysts, solvents, concentrations, temperatures etc. to achieve the best results.
Catalysts
The catalysts used for ROMP are the same catalysts used for olefin metathesis. However, one has to be a little more careful when selecting a ROMP catalyst. If the catalyst is too active, it can metathesize the unstrained olefinic bonds in the growing polymer chain (a process called "back-biting"), thereby reducing the molecular weight and increasing the molecular weight distribution (polydispersity). For example, consider the synthesis of polyacetylene by ROMP of cyclooctatetraene shown below. Backbiting occurs when the growing polymer chain can orient to undergo an intramolecular metathesis and generate benzene, a thermodynamic sink. To prevent backbiting in this or other systems you must make sure your catalyst does not react with internal olefins!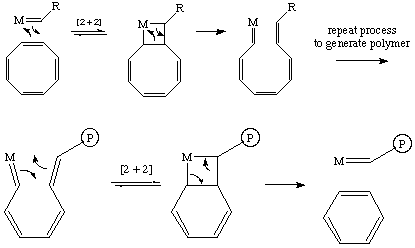
When the reaction is complete, there are two methods for cleaving the polymer from the metal center:
- Reaction with aldehyde. Many metathesis catalysts react with aldehydes in a [2 + 2] fashion just as they do with olefins. The product is a metal oxo and an olefin (or polymer) capped with the former aldehyde functionality. Often a large excess (100 equiv) of aldehyde is used. The cleaved polymer can then be separated from the catalyst by precipitation with methanol.
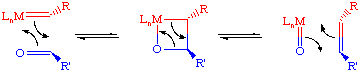
- Chain transfer. Reaction with several equivalents of diene is another way of cleaving the polymer chain. The advantage of this method is that the cleavage does not deactivate the catalyst, permitting additional aliquots of monomer to be polymerized. However, one does have to worry about broadening the MW distribution:

Ring Closing Metathesis
As the name implies, this is the reverse of the ROMP reaction. In order to make it work, the ring being formed can not have appreciable ring strain. As the RCM and ROMP processes involve equilibria, the RCM reaction sometimes involves running the experiment at low dilution so that most of the reactions are intra- rather than intermolecular. In the examples below, removal of the volatile byproduct drives the equilibrium to the ring-closed product. In addition, the catalysts are selected to have good reactivity with terminal olefins, but low reactivity with internal ones. The starting materials for each reaction are shown in the top row and the products in the bottom: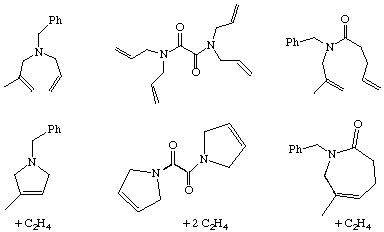
Further Reading

[Index] [Keyword Search] [Books & Software]
[ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.












